N-苯基-1-(2-苯乙基)哌啶-4-胺(枸橼酸芬太尼杂质I) 标准品
| 储存条件 | RT,避光 |
| 规格 | 50mg |

N-苯基-1-(2-苯乙基)哌啶-4-胺(枸橼酸芬太尼杂质I) 标准品
| 储存条件 | RT,避光 |
| 规格 | 50mg |
丙烯酰哌啶胺

| 有效期 | 2年 |
| MDL | MFCD30343868 |
| 别名 | EOS-61890 |
| 英文名称 | PF-06651600 |
| CAS | 1792180-81-4 |
| 分子式 | C15H19N5O |
| 分子量 | 285.34 |
| 储存条件 | 2-8℃ |
| 纯度 | ≥98% |
| 外观(性状) | White to off-white (Solid) |
| 单位 | 瓶 |
| 生物活性 | PF-06651600 is a potent JAK3-selective inhibitor (IC50: 33.1 nM).[1-2] |
| In Vitro | PF-06651600 inhibited JAK3 kinase activity with an IC50 of 33.1 nM but without activity (IC50 > 10 000 nM) against JAK1, JAK2, and TYK2. In total lymphocytes in human whole blood, PF-06651600 inhibited the phosphorylation of STAT5 elicited by IL-2, IL-4, IL-7, and IL-15 with IC50 values of 244, 340, 407, and 266 nM, respectively, and it inhibited the phosphorylation of STAT3 elicited by IL-21 with an IC50 of 355 nM [1]. |
| In Vivo | In the rat adjuvant-induced arthritis (AIA) model, PF-06651600 reduced paw swelling with an unbound EC50 of 169 nM. Similarly, PF-06651600 significantly reduced disease severity in the experimental autoimmune encephalomyelitis21,22 (EAE) mouse model when dosed either therapeutically at 30 or 100 mg/kg or prophylactically at 20 and 60 mg/kg [1]. |
| 激酶实验 | His-tagged recombinant human TYK2 kinase domain was expressed in SF21/baculovirus and purified using a two-step affinity (Ni-NTA) and size-exclusion (SEC S200) purification method.Test compounds were solubilized in DMSO to a stock concentration of 30 mM.Compounds were diluted in DMSO to create an 11-point half log dilution series with a top concentration of 600 μM.The test compound plate also contained positive control wells containing a known inhibitor to define 100% inhibition and negative control wells containing DMSO to define no inhibition.The compound plates were diluted 1 to 60 in the assay,resulting in a final assay compound concentration range of 10 μM to 100 pM and a final assay concentration of 1.7% DMSO.Test compounds and controls solubilized in 100% DMSO were added (250 nL) to a 384 well polypropylene plate (Matrical) using a non contact acoustic dispenser.Kinase assays were carried out at room temperature in a 15 μL reaction buffer containing 20 mM HEPES,pH 7.4,10 mM magnesium chloride,0.01% bovine serum albumin (BSA),0.0005% Tween 20 and 1mM Dithiothreitol (DTT).Reaction mixtures contained 1 μM of a fluorescently labeled synthetic peptide,at a concentration less than the apparent Michaelis-Menten constant (Km) (5FAM-KKSRGDYMTMQID for JAK1 and TYK2 and FITC-KGGEEEEYFELVKK for JAK2 and JAK3).Reaction mixtures contained adenosine triphosphate (ATP) at either a level equal to the apparent Km for ATP (40 μM for JAK1,4 μM for JAK2,4 μM for JAK3 and 12 μM for TYK2) or at 1 mM ATP.Compound was added to the buffer containing ATP and substrate and immediately after this step the enzyme was added to begin the reaction.The assays were stopped with 15 μL of a buffer containing 180 mM HEPES,pH=7.4,20 mM EDTA,0.2% Coating Reagent,resulting in a final concentration of 10 mM EDTA,0.1% Coating Reagent and 100 mM HEPES,pH=7.4. |
| SMILES | C=CC(N1[C@@H](C)CC[C@@H](NC2=C3C(NC=C3)=NC=N2)C1)=O |
| 靶点 | JAK |
| 动物实验 | Human CD4+ T cells were purified from buffy coat with RosetteSep CD4+ T Cell Enrichment Cocktail and skewed for 6 days with cytokine cocktails (25 ng/mL of IL-6, 25 ng/mL of IL-23, 12.5 ng/mL of IL-1β, 25 ng/mL of IL21, 5 ng/mL of TGFβ1, 10 μg/ml of anti-CD3 antibody (pre-coated on plate surface) and 1 μg/mL of anti-CD28 antibody) in the presence of JAK inhibitors at 10 different concentrations. Supernatants were harvested and the concentrations of IL-17A were determined with MSD assay following the protocol provided by the manufacturer. To study the effect of PF-06651600 on Th17 cells post-differentiation, skewed Th17 cells were washed, rested with X-VIVO 15 medium for overnight and resuspended in medium containing the same concentrations of cytokines as during skewing but without anti-CD3 or anti-CD28 antibodies, in the presence of PF-06651600 at 10 different concentrations for 2 additional days. On Day 9, supernatant was harvested from each well and IL-17A was determined as described above [1]. |
| 细胞实验 | The effect of JAK3 inhibition by PF-06651600 was evaluated in vivo using a therapeutic dosing paradigm in a rat adjuvant-induced arthritis. The efficacy of this molecule was evaluated in three separate studies using successively lower doses. Arthritis was induced by immunization of female Lewis rats (8 to 10 weeks old) via intradermal injection at the base of the tail with complete Freund’s adjuvant with three 50 μL injections (15 mg/mL Mycobacterium tuberculosis) in incomplete Freund’s adjuvant. Seven days after the initial immunization, the baseline hind paw volume of the immunized rats was measured via plethysmograph. The rats were monitored daily for signs of arthritis including change in body weight and hind paw volume measurement. When individual hind paw volume measurements indicated an increase of 0.2 mL (or greater) in a single hind paw, animals were randomly assigned to a treatment group. Daily treatment with PF-06651600 was administered via oral gavage. Treatment groups for Experiment 1 were: 80, 15, or 6 mg/kg or vehicle (2% Tween 80 /0.5% methylcellulose/deionized water). Treatment groups for Experiment 2 were: 30, 10, and 3 mg/kg or vehicle (0.5% methylcellulose / de-ionized water/ 1 mEQ hydrochloric acid). Treatment groups for Experiment 3 were: 10, 1, 0.3 and 0.1 mg/kg or vehicle (0.5% methylcellulose/de-ionized water/ 1 mEQ hydrochloric acid). Dosing began once individuals were enrolled into respective groups. Treatment continued for 7 days. At the conclusion of the study, whole blood was taken at 15 minutes post-dose (peak concentration in plasma) for analysis of STAT phosphorylation, and plasma was taken for exposure concentration in PF-06651600 dosed groups [1]. |
| 数据来源文献 | [1]. Telliez JB, et al. Discovery of a JAK3-Selective Inhibitor: Functional Differentiation of JAK3-Selective Inhibition over pan-JAK or JAK1-Selective Inhibition. ACS Chem Biol. 2016 Dec 16;11(12):3442-3451. [2]. Thorarensen A, et al. Design of a Janus Kinase 3 (JAK3) Specific Inhibitor 1-((2S,5R)-5-((7H-Pyrrolo[2,3-d]pyrimidin-4-yl)amino)-2-methylpiperidin-1-yl)prop-2-en-1-one (PF-06651600) Allowing for the Interrogation of JAK3 Signaling in Humans. J Med Chem. 2017 Mar 9;60(5):1971-1993. |
| 规格 | 5mg 10mg |
是一种新颖,有效,选择性和不可逆的JAK3抑制剂
酞胺哌啶酮 标准品
| 规格 | 50mg |
3-氨基-2-哌啶酮
| 英文名称 | 3-amino-2-Piperidinone |
| CAS | 34294-79-6 |
| 储存条件 | 2-8℃ |
| 单位 | 瓶 |
| 规格 | 25mg |
氟哌啶醇 标准品
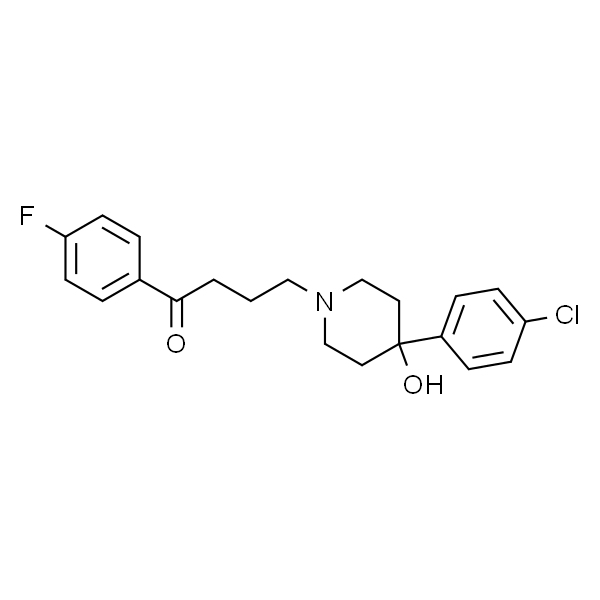
| 英文名称 | Haloperidol |
| CAS | 52-86-8 |
| 分子式 | C21H23ClFNO2 |
| 分子量 | 375.86 |
| 规格 | 50mg |
2-哌啶甲酸
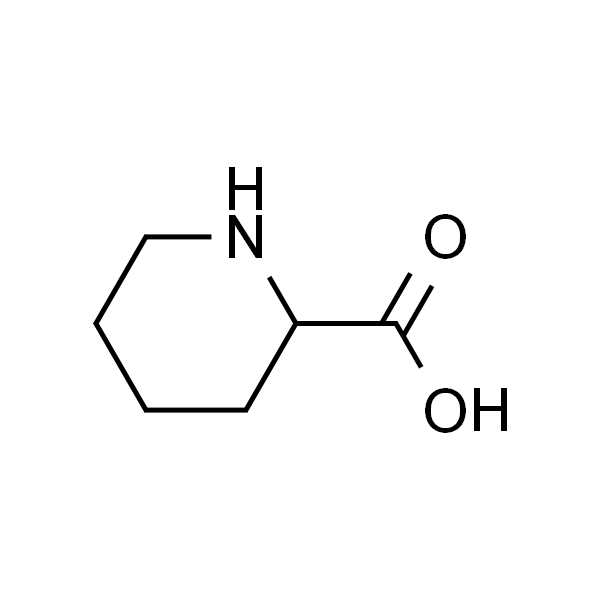
| 有效期 | 2年 |
| MDL | MFCD00064347 |
| EC | EINECS 208-616-3 |
| InChIKey | HXEACLLIILLPRG-UHFFFAOYSA-N |
| InChI | InChI=1S/C6H11NO2/c8-6(9)5-3-1-2-4-7-5/h5,7H,1-4H2,(H,8,9) |
| PubChem CID | 849 |
| 别名 | 哌啶酸,哌啶-2-甲酸,呢可酸 |
| 英文名称 | Pipecolic Acid |
| CAS | 535-75-1 |
| 分子式 | C6H11NO2 |
| 分子量 | 129.16 |
| 储存条件 | 2-8℃ |
| 纯度 | ≥98% |
| 外观(性状) | White to off-white (Solid) |
| 单位 | 瓶 |
| SMILES | O=C(O)C1CCCCN1 |
| 靶点 | Others |
| 规格 | 100mg 500mg |
是赖氨酸的代谢产物。
4-羟基-TEMPO
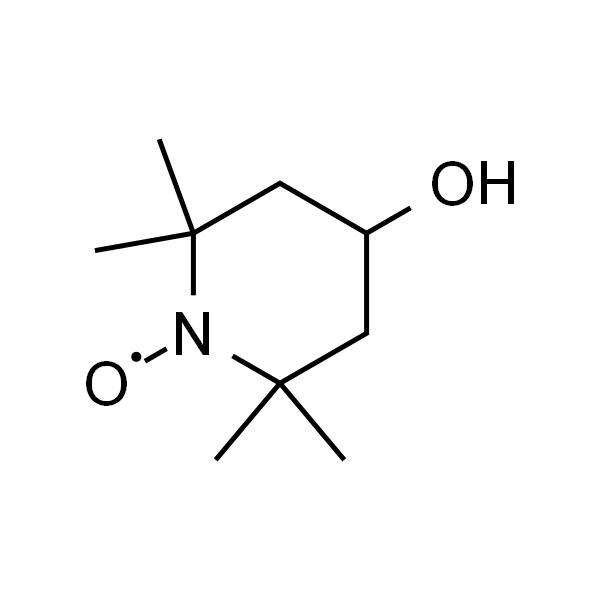
| 有效期 | 2年 |
| MDL | MFCD00006478 |
| EC | EINECS 218-761-4 |
| 别名 | 4-羟基哌啶醇氧自由基; 哌啶醇氧化物; 4-Hydroxy-TEMPO; Tanol; TMPN; 氮氧自由基哌啶醇;4-羟基-2,2,6,6-四甲基哌啶-1-氧自由基; 4-羟基-TEMPO; 阻聚剂701 |
| 英文名称 | Tempol |
| CAS | 2226-96-2 |
| 分子式 | C9H18NO2 |
| 分子量 | 172.24 |
| 储存条件 | 2-8℃ |
| 纯度 | ≥98% |
| 外观(性状) | Solid |
| 单位 | 瓶 |
| 生物活性 | Tempol 是一种超氧化物歧化酶 (SOD) 类似物,可有效中和活性氧 (ROS)。[1-2] |
| In Vitro | Tempol显着减弱H2O2介导的线粒体呼吸减少和大鼠PT细胞LDH释放的增加,表明细胞损伤和死亡减少。 Tempol的有益作用类似于使用Fe2 +螯合剂DEF获得的作用。然而,DEF和Tempol的共同给药不会产生任何针对肾缺血/再灌注损伤或抗氧化应激介导的PT细胞损伤/死亡的额外有益作用[2]。 |
| In Vivo | SOD模拟Tempol能够模仿白藜芦醇对心脏功能的影响。通过强饲法每天施用Tempol。与口服载体(VEH)相比,用Met或Tmp处理的小鼠具有降低的PR和QTc间隔以及增加的心率。这些结果与通过RSV治疗获得的结果相似。描述了个体小鼠的治疗前和治疗后的概况[1]。 Tempol是一种可透过膜的自由基清除剂,可减轻大鼠氧化应激介导的肾功能障碍和损伤。 Tempol显着降低肾缺血/再灌注产生的尿素,肌酐,γGT,AST,NAG和FENa的增加,表明肾功能和损伤均得到改善。 Tempol还显着降低肾脏MPO活性和MDA水平,分别表明PMN浸润和脂质过氧化减少。 Tempol减少了与缺血/再灌注相关的肾损伤的组织学证据,并导致硝基酪氨酸和PARS染色显着减少,表明亚硝化和氧化应激减少[2]。 |
| SMILES | CC1(CC(CC(N1[O])(C)C)O)C |
| 靶点 | ROS |
| 动物实验 | 小鼠[1]使用雌性或雄性BALB / c小鼠(5-7周龄)。用102克血液trypomastigote形式的克氏锥虫的I型哥伦比亚菌株腹膜内(ip)感染小鼠。通过ip注射15mg / kg反式白藜芦醇(10%乙醇/ PBS),媒介物(10%乙醇/ PBS),5mg / Kg EX527(每天进行CCC(60dpi)建立30天后进行治疗) 0.1%DMSO),或口服40mg / Kg白藜芦醇(10%乙醇-PBS),500mg / kg二甲双胍(溶于水),100mg / kg Tempol(溶于水),苯并咪唑(25mg / Kg) ,溶于水)和载体(水或10%乙醇-PBS)。大鼠[2]使用重量为230至320g的83只雄性Wistar大鼠。完成外科手术后,将动物随机分配到8组。在再灌注开始前一分钟,动物接受推注注射载体(盐水,4mL / kg,IV),Tempol(30mg / kg,在盐水中,IV),DEF(40mg / kg,在盐水中,IV)。 ,或DEF(40mg / kg盐水,IV)与Tempol(30mg / kg盐水,IV)组合。然后相应的组在整个再灌注期间连续输注以下一种:载体(盐水,4 mL / kg / h,静脉注射),Tempol(30 mg / kg / h盐水,静脉注射),DEF(40 mg)盐水中的/ kg / h,IV),或组合的Tempol和DEF(分别为30和40mg / kg / h,在盐水,IV中)。为了阐明Tempol或DEF对假手术大鼠的心血管血流动力学和器官功能的影响,各组动物接受本文前面所述的治疗和概述。体内施用的Tempol浓度基于我们先前证明的在体内大鼠模型中提供针对心肌缺血/再灌注损伤的显着保护的那些浓度。类似地,使用的DEF浓度与我们先前用于在体内大鼠和兔模型中提供针对肝缺血/再灌注损伤的显着保护的浓度相同。 |
| 细胞实验 | 为了研究与Tempol共同施用Tempol,DEF和DEF对H2O2介导的细胞损伤和死亡的影响,PT细胞的汇合培养物与Tempol(0.03至10mM),DEF(0.03)预孵育(在37℃下10分钟)。与Tempol(3mM)组合的DEF(3mM)至10mM)或DEF(3mM)。 Tempol和DEF的浓度范围基于此实验室先前显示的那些,以减少H2O2介导的细胞损伤和死亡(1)培养的大鼠心肌成肌细胞(Tempol)和(2)大鼠PT细胞的原代培养物(DEF) )。然后将PT细胞培养物与H 2 O 2(1mM)一起温育4小时,之后评估细胞损伤和死亡。完成孵育后,使用本文后面描述的分光光度测定法评估细胞损伤和死亡[2]。 |
| 数据来源文献 | [1]. Vilar-Pereira G, et al. Resveratrol Reverses Functional Chagas Heart Disease in Mice. PLoS Pathog. 2016 Oct 27;12(10):e1005947.
[2]. Chatterjee PK, et al. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000 Aug;58(2):658-73 |
| 规格 | 10mM*1mL in Water 10mM*1mL in DMSO 100mg 200mg 500mg |
Tempol 是一种超氧化物歧化酶 (SOD) 类似物,可有效中和活性氧 (ROS)。
DL-Pipecolinic acid 六氢吡啶羧酸 标准品
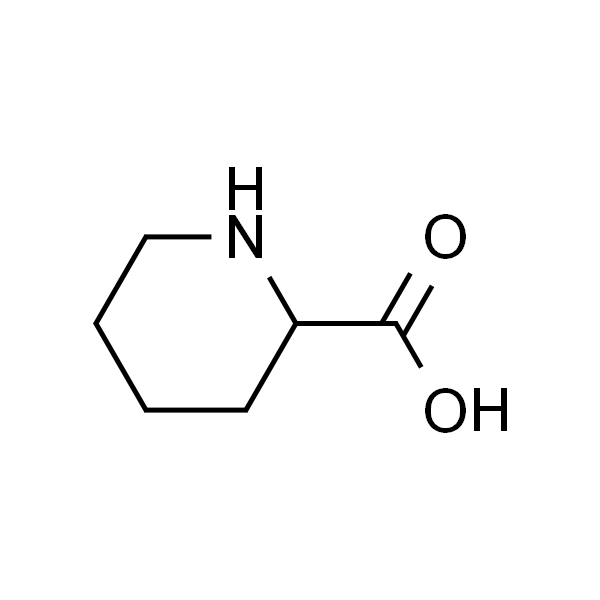
| EC | EINECS 208-616-3 |
| MDL | MFCD00064347 |
| 别名 | Pipecolic acid;(±)-哌啶-2-羧酸;六氢吡啶-alpha-羧酸;2-甲酸哌啶;哌啶-2-甲酸;DL-哌啶-2-甲酸;DL-2-哌啶甲酸;哌啶甲酸;2-哌啶甲酸;呢可酸;DL-2-哌啶酸;DL-Homoproline |
| 英文名称 | DL-Pipecolinic acid |
| CAS | 535-75-1 |
| 分子式 | C6H11NO2 |
| 分子量 | 129.16 |
| 储存条件 | 2-8℃ |
| 纯度 | HPLC≥98% |
| 单位 | 瓶 |
| SMILES | O=C(O)C1CCCCN1 |
| 规格 | 20mg |
本品为分析标准品。
氨鲁米特 标准品
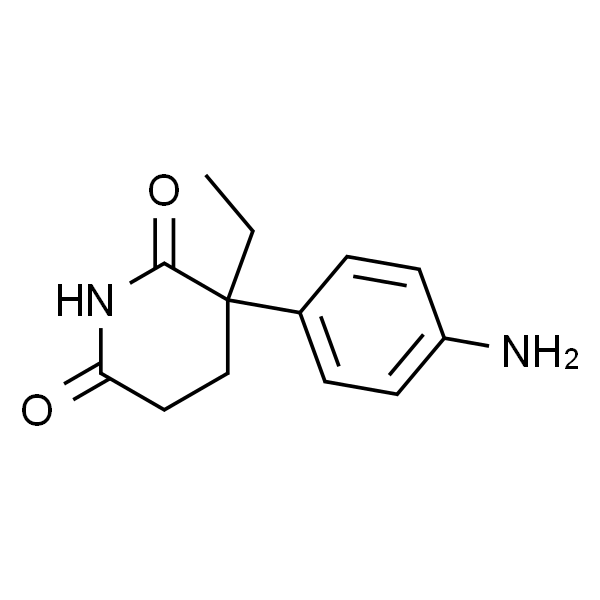
| 英文名称 | DL-Aminoglutethimide |
| CAS | 125-84-8 |
| 分子式 | C13H16N2O2 |
| 分子量 | 232.28 |
| 储存条件 | 2-8℃ |
| 规格 | 50mg |