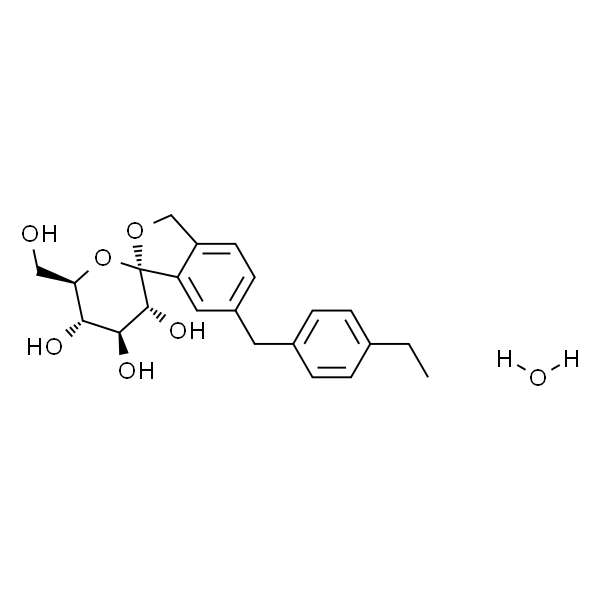
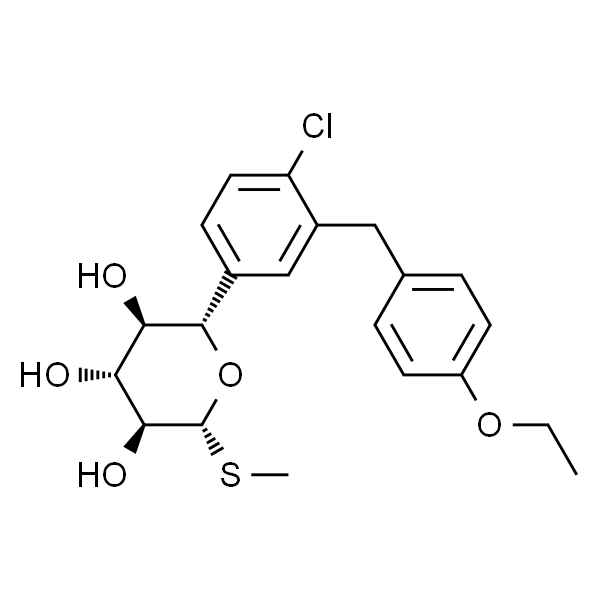
埃格列净
| 有效期 | 2年 |
| MDL | MFCD21609259 |
| 别名 | PF-04971729 |
| 英文名称 | Ertugliflozin |
| CAS | 1210344-57-2 |
| 分子式 | C22H25ClO7 |
| 分子量 | 436.88 |
| 储存条件 | -20°C |
| 纯度 | ≥98% |
| 外观(性状) | White Powder |
| 单位 | 瓶 |
| 生物活性 | Ertugliflozin (PF-04971729) 是有效的、选择性的、具有口服活性的钠离子依赖的葡萄糖协同转运蛋白2 (SGLT2) 的抑制剂。其对h-SGLT2 的 IC50 值为0.877 nM。是一种治疗2型糖尿病的临床药物[1-3]。 |
| IC50 | 0.877 nM (h-SGLT2)[1-3]. |
| In Vitro | Ertugliflozin (it is claimed) has a 2000-fold increase in selectivity for human SGLT2 over SGLT1 (IC50: SGLT2 = 0.877 nM vs SGLT1 = 1960 nM) in vitro[3]。 |
| In Vivo | Ertugliflozin is rapidly absorbed in preclinical species after oral administration, and it is characterized by low clearance (excreted in the urine in preclinical species) and a moderate steady-state distribution volume. There is low potential for pharmacokinetic interaction of ertugliflozin. Ertugliflozin is well absorbed in humans and eliminated largely via glucuronidation. Ertugliflozin improved glycemic control, body weight and blood pressure in patients with T2DM suboptimally controlled by metformin, and is well-tolerated[3]。 |
| SMILES | ClC1=CC=C([C@]23O[C@@](CO)(CO3)[C@@H](O)[C@H](O)[C@H]2O)C=C1CC4=CC=C(OCC)C=C4 |
| 靶点 | SGLT |
| 数据来源文献 | [1]. Mascitti V, et al. Discovery of a clinical candidate from the structurally unique dioxa-bicyclo[3.2.1]octane class of sodium-dependent glucose cotransporter 2 inhibitors. J Med Chem. 2011 Apr 28;54(8):2952-60. [2]. Miao Z, et al. Pharmacokinetics, metabolism, and excretion of the antidiabetic agent ertugliflozin (PF-04971729) in healthy male subjects. Drug Metab Dispos. 2013 Feb;41(2):445-56. [3]. Jiang M, Steyger PS. An evaluation of US patent 2015065565 (A1) for a new class of SGLT2 inhibitors for treatment 1 of type II diabetes mellitus. Expert Opin Ther Pat. 2015;25(11):1349-1352. |
| 规格 | 1mg 5mg |
是有效的、选择性的钠离子依赖的葡萄糖协同转运蛋白2 (SGLT2) 的抑制剂。可用于2型糖尿病的相关研究。